Is GLOPERBA® (colchicine) right for your patients with gout?
GLOPERBA is the first and only liquid colchicine
Simplified dose adjustment allows you to individualize therapy, patient to patient
- GLOPERBA is indicated for the prophylaxis of gout flares in adults1
- Unique liquid oral solution1,2
- Allows for simple and precise titration when necessary
- Pleasant cherry taste
GLOPERBA delivers the proven efficacy of colchicine
Colchicine prophylaxis* + urate-lowering therapy significantly reduces the frequency of gout flares vs urate-lowering therapy alone3
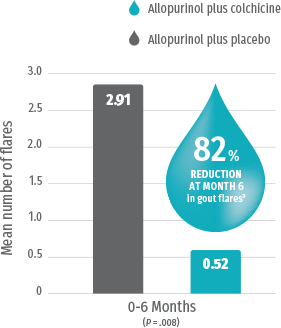
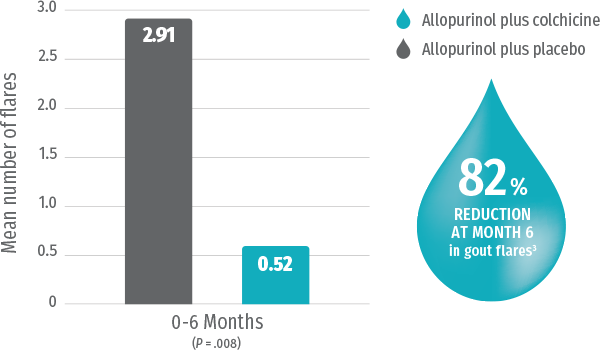
Study Design Randomized, prospective, double-blind, placebo-controlled trial evaluating the safety and efficacy of colchicine in the management of gout (N=43). Patients starting allopurinol were randomized to receive colchicine 0.6 mg twice a day or placebo. Average baseline serum urate levels were 9.49 mg/dL in the colchicine group.3
GLOPERBA is an approved NDA based on the demonstrated bioequivalence of a 0.6-mg colchicine tablet with 5 mL (0.6 mg) of GLOPERBA solution. The efficacy data presented here are from the study of 0.6 mg of colchicine for the prophylaxis of gout flares.
As a liquid, GLOPERBA was designed for the real-world gout patient, who is likely to have comorbidities
Patients who have gout may require colchicine titration, especially those with kidney and liver problems
NHANES: Common comorbidities in patients with gout4
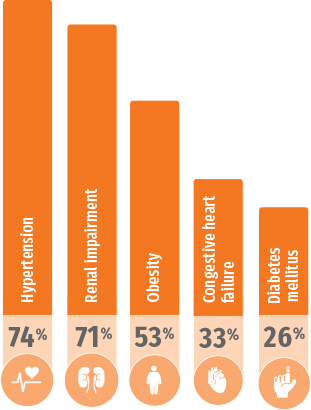
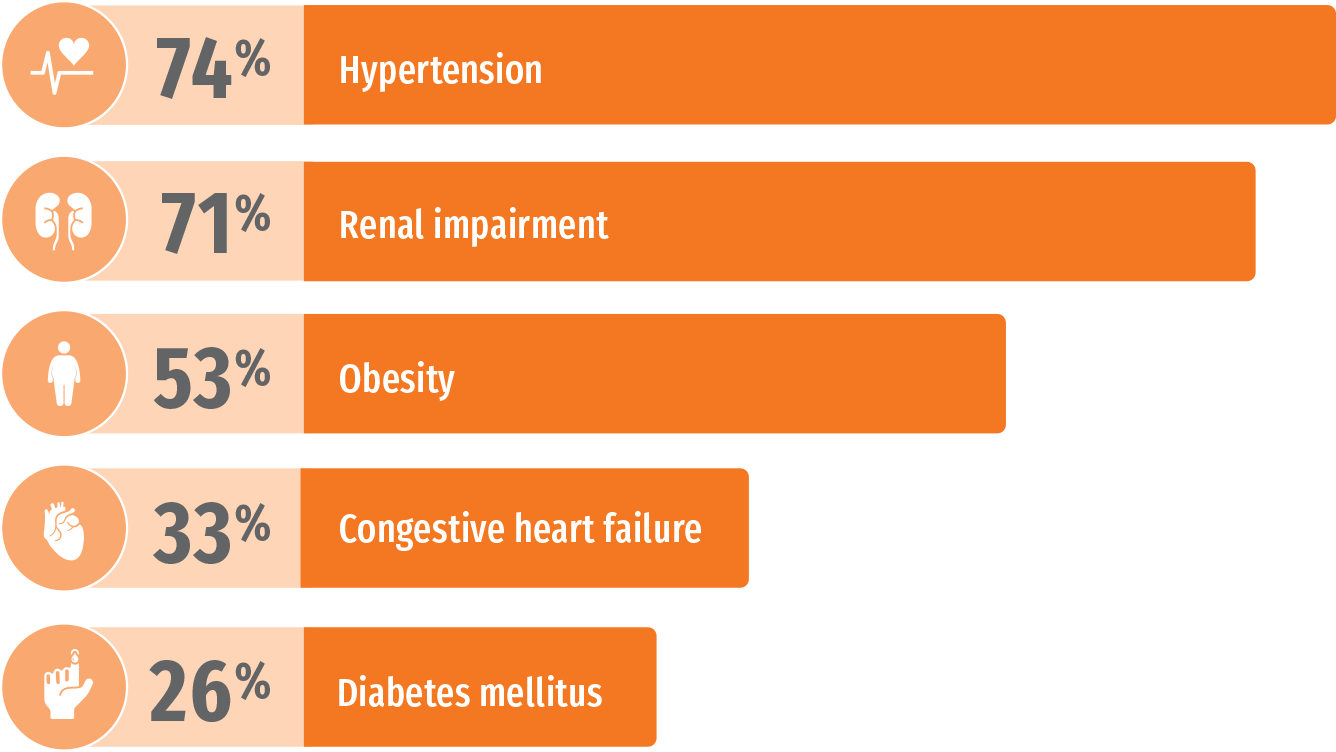
Estimated prevalence of major comorbidities associated with gout using data from 5707 participants aged 20 years or older in the National Health and Nutrition Examination Survey (NHANES) 2007-2008.4
GLOPERBA was designed for easy administration and dose titration1
In clinical trials, gout-flare prophylaxis with colchicine was administered for a 6-month period3
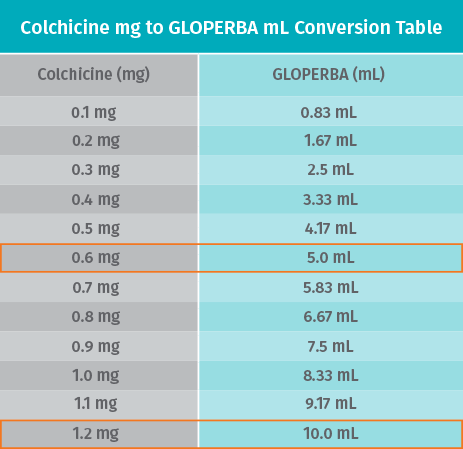
- Recommended dose of GLOPERBA is 0.6 mg (5 mL) once or twice daily1
- Maximum dose is 1.2 mg daily1
- Dose modifications or adjustments should be considered in patients with renal dysfunction or hepatic impairment1
- GLOPERBA can be taken with or without food1
- Dosing syringe facilitates precise dosing by patients based on your directions

The safety profile of colchicine is well characterized
The use of colchicine in the management of chronic gout has been studied for decades5
The safety of colchicine as a part of gout-flare prophylaxis was established in 2 randomized, placebo-controlled trials1
The safety of colchicine is backed by decades of real-world clinical experience5
The most commonly reported adverse reactions with colchicine are gastrointestinal symptoms, including diarrhea, nausea, vomiting, and abdominal pain.1
Important Safety Information for GLOPERBA® (colchicine)
- Colchicine 0.6 mg oral solution is contraindicated in patients with renal or hepatic impairment who are currently prescribed drugs that inhibit both P-gp and CYP3A4. Combining these dual inhibitors with colchicine in patients with renal or hepatic impairment has resulted in life-threatening or fatal colchicine toxicity. Patients with both renal and hepatic impairment should not be given GLOPERBA.
- Fatal overdoses have been reported with colchicine in adults and children. Keep GLOPERBA out of the reach of children.
- Blood dyscrasias, such as myelosuppression, leukopenia, granulocytopenia, thrombocytopenia, and aplastic anemia, have been reported with colchicine used in therapeutic doses.
- Monitor for toxicity and, if present, consider lowering the dose, temporary interruption, or discontinuation of colchicine.
- Neuromuscular toxicity and rhabdomyolysis may occur with chronic treatment with colchicine in therapeutic doses, especially in combination with other drugs known to cause this effect. Patients with impaired renal function and elderly patients (including those with normal renal and hepatic function) are at increased risk. Consider lowering the dose, temporary interruption, or discontinuation of GLOPERBA.
- The most commonly reported adverse reactions with colchicine are gastrointestinal symptoms, including diarrhea, nausea, vomiting, and abdominal pain.
Indication
GLOPERBA 0.6 mg oral solution is indicated for prophylaxis of gout flares in adults. The safety and effectiveness of GLOPERBA for acute treatment of gout flares during prophylaxis has not been studied.
GLOPERBA is not an analgesic medication and should not be used to treat pain from other causes.
You are encouraged to report negative side effects of prescription drugs to the FDA. To report suspected adverse reactions, visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please see Full Prescribing Information for GLOPERBA.
References: 1. GLOPERBA [package insert]. Alpharetta, GA: Avion Pharmaceuticals, LLC; 2019. 2. US Food and Drug Administration. Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. https://www.accessdata.fda.gov/scripts/cder/ob/index.cfm. Updated January 2020. Accessed April 7, 2020. 3. Borstad GC, Bryant LR, Abel MP, Scroggie DA, Harris MD, Alloway JA. Colchicine for prophylaxis of acute flares when initiating allopurinol for chronic gouty arthritis. J Rheumatol. 2004;31(12):2429-2432.
References: 1. Eggebeen AT. Gout: an update. Am Fam Physician. 2007;76(6):801-808. 2. Bobulescu IA, Moe OW. Renal transport of uric acid: evolving concepts and uncertainties. Adv Chronic Kidney Dis. 2012;19(6):358-371. 3. Ragab G, Elshahaly M, Bardin T. Gout: an old disease in new perspective—a review. J Adv Res. 2017;8(5):495-511. 4. Finn WF. Kidney disease and gout: the role of the innate immune system. Open Urol Nephrol J. 2016;9(suppl 1):12-21. 5. Cronstein BN, Terkeltaub R. The inflammatory process of gout and its treatment. Arthritis Res Ther. 2006;8(1):S3. 6. Schlesinger N. Treatment of chronic gouty arthritis: it is not just about urate-lowering therapy. Semin Arthritis Rheum. 2012;42(2):155-165. 7. Bădulescu M, Macovei L, Rezuş E. Acute gout attack with normal serum uric acid levels. Rev Med Chir Soc Med Nat Iasi. 2014;118(4):942-945.
References: 1. Borstad GC, Bryant LR, Abel MP, Scroggie DA, Harris MD, Alloway JA. Colchicine for prophylaxis of acute flares when initiating allopurinol for chronic gouty arthritis. J Rheumatol. 2004;31(12):2429-2432. 2. Schlesinger N. Treatment of chronic gouty arthritis: it is not just about urate-lowering therapy. Semin Arthritis Rheum. 2012;42(2):155-165. 3. FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020;72(6):744-760. 4. GLOPERBA [package insert]. Alpharetta, GA: Avion Pharmaceuticals, LLC; 2019.
References: 1. GLOPERBA [package insert]. Alpharetta, GA: Avion Pharmaceuticals, LLC; 2019. 2. US Food and Drug Administration. Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. https://www.accessdata.fda.gov/scripts/cder/ob/index.cfm. Revised January 17, 2020. Accessed February 7, 2020. 3. Borstad GC, Bryant LR, Abel MP, Scroggie DA, Harris MD, Alloway JA. Colchicine for prophylaxis of acute flares when initiating allopurinol for chronic gouty arthritis. J Rheumatol. 2004;31:2429-2432. 4. Zhu Y, Pandya BJ, Choi HK. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007-2008. Am J Med. 2011;125:679-687. 5. Slobodnick A, Shah B, Pillinger MH, Krasnokutsky S. Colchicine: old and new. Am J Med. 2015;128(5):461-470.
References: 1. US Food and Drug Administration. Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. https://www.accessdata.fda.gov/scripts/cder/ob/index.cfm. Current through December 2020. Accessed December 7, 2020. 2. GLOPERBA [package insert]. Alpharetta, GA: Avion Pharmaceuticals, LLC; 2019.

