Are you using colchicine for gout-flare prophylaxis?
Inflammation prophylaxis is a crucial component of the management of gout1


The clinical case for a 6-month course of inflammation prophylaxis with colchicine in chronic gout:
- Initiation of urate-lowering therapy may trigger breakthrough gout attacks with inflammation2
- Inflammation is a key hallmark of recurrent gout2
- In clinical studies, 6 months of inflammation prophylaxis with colchicine has been shown to prevent gout flares better than urate-lowering therapy alone1
Colchicine is thought to work by disrupting the gout inflammatory cascade in 2 key ways4
Blocking
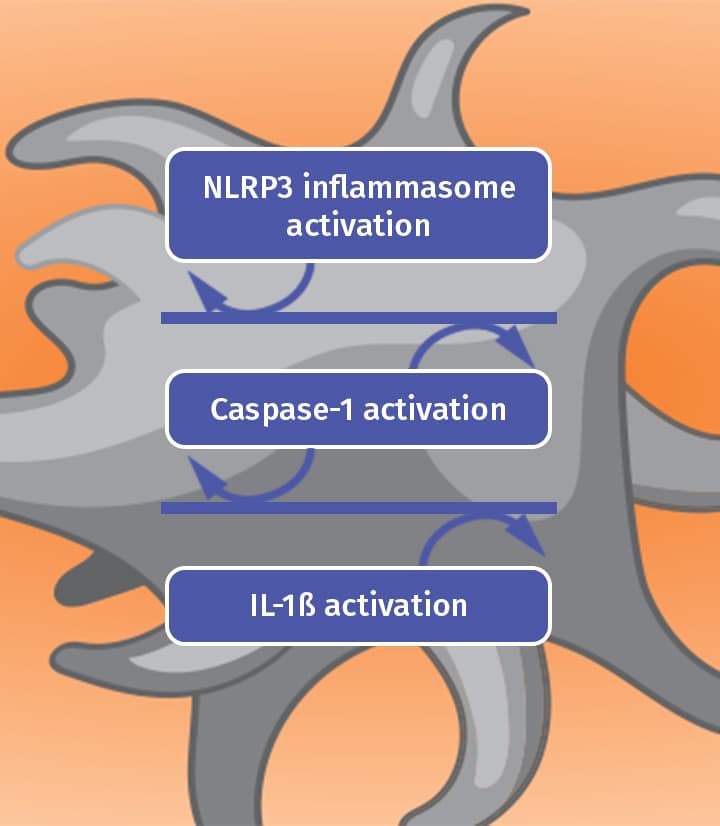
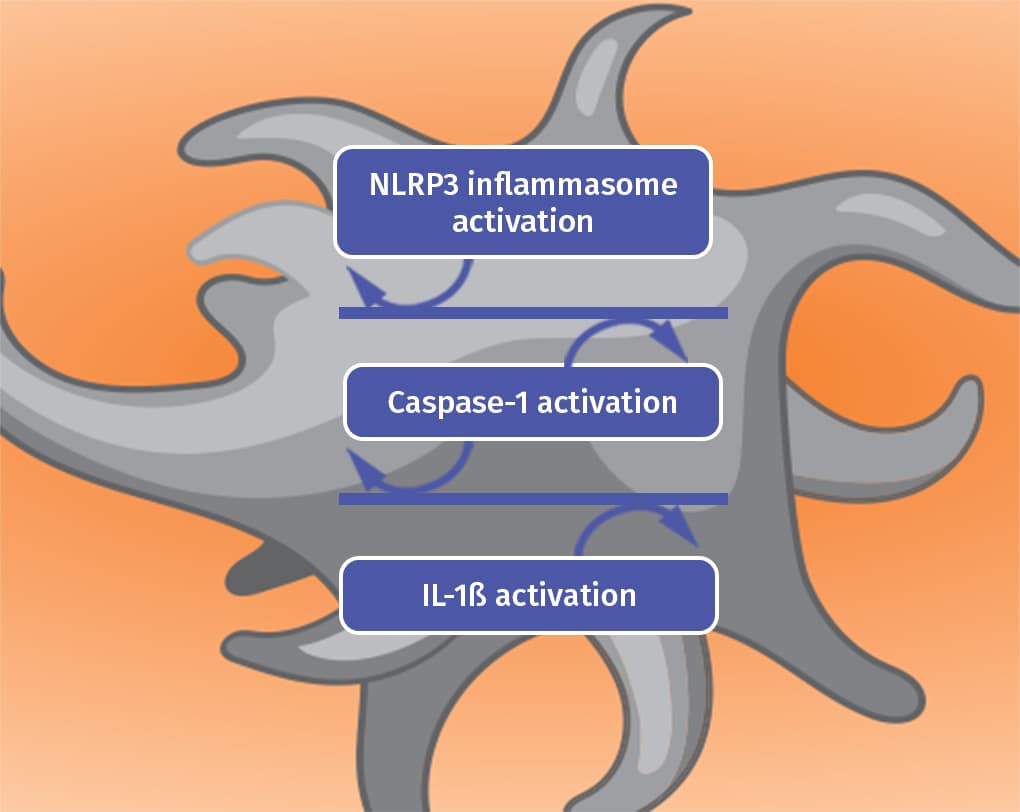
Colchicine blocks the inflammasome complex that mediates interleukin activation.4
IL-1ß: Interleukin-1 beta.Prevention
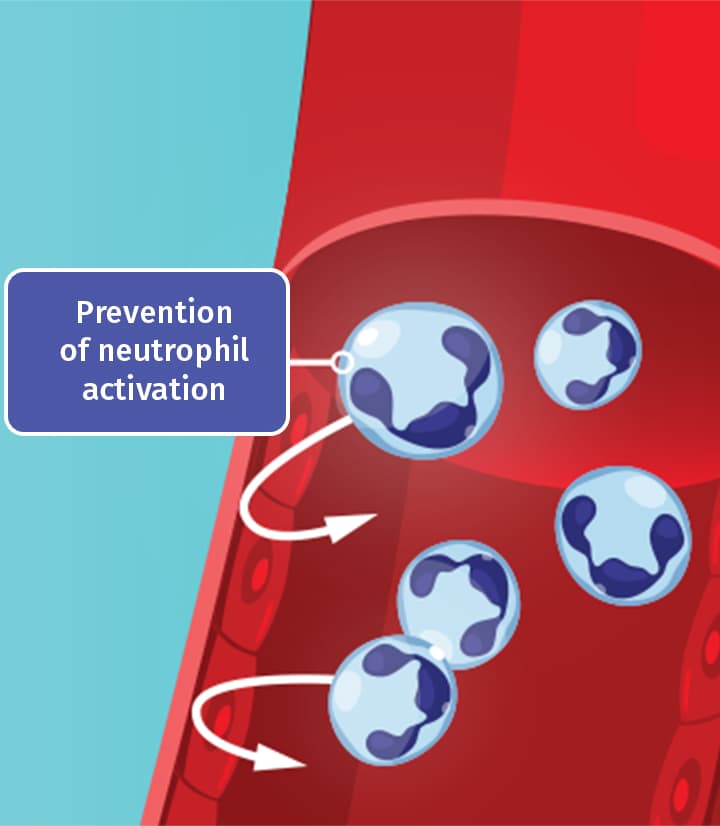
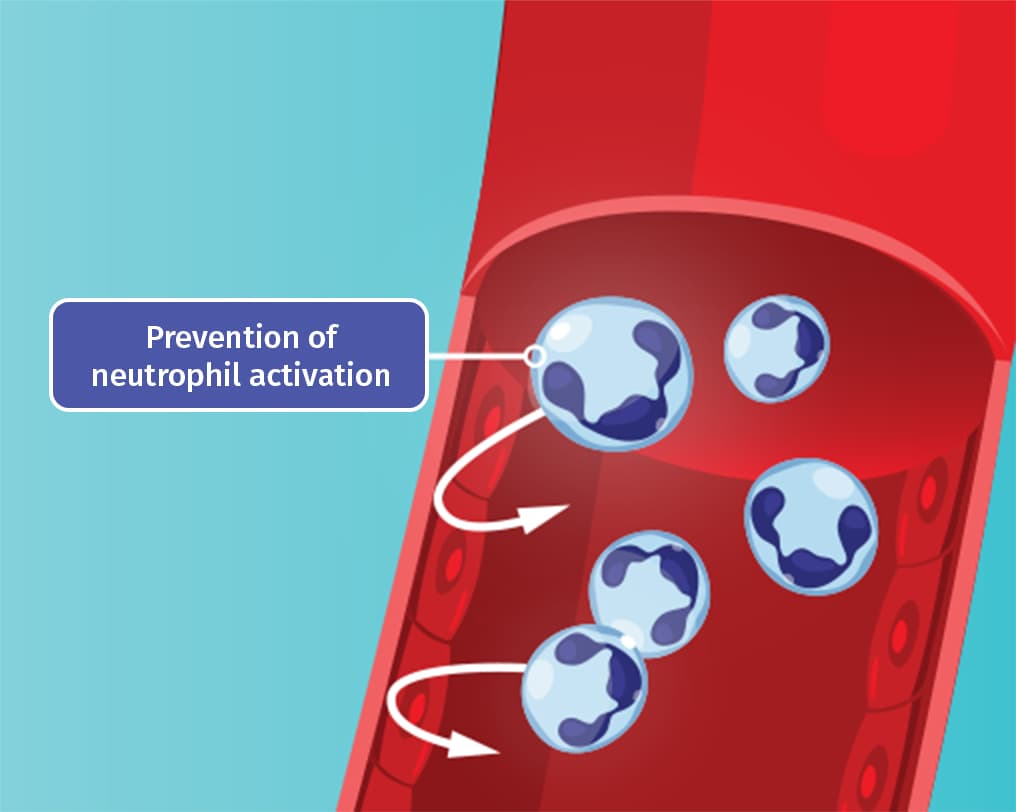
By inhibiting polymerization of tubulin into microtubules, colchicine prevents neutrophil activation and migration to sites of inflammation.4
Important Safety Information for GLOPERBA® (colchicine)
- Colchicine 0.6 mg oral solution is contraindicated in patients with renal or hepatic impairment who are currently prescribed drugs that inhibit both P-gp and CYP3A4. Combining these dual inhibitors with colchicine in patients with renal or hepatic impairment has resulted in life-threatening or fatal colchicine toxicity. Patients with both renal and hepatic impairment should not be given GLOPERBA.
- Fatal overdoses have been reported with colchicine in adults and children. Keep GLOPERBA out of the reach of children.
- Blood dyscrasias, such as myelosuppression, leukopenia, granulocytopenia, thrombocytopenia, and aplastic anemia, have been reported with colchicine used in therapeutic doses.
- Monitor for toxicity and, if present, consider lowering the dose, temporary interruption, or discontinuation of colchicine.
- Neuromuscular toxicity and rhabdomyolysis may occur with chronic treatment with colchicine in therapeutic doses, especially in combination with other drugs known to cause this effect. Patients with impaired renal function and elderly patients (including those with normal renal and hepatic function) are at increased risk. Consider lowering the dose, temporary interruption, or discontinuation of GLOPERBA.
- The most commonly reported adverse reactions with colchicine are gastrointestinal symptoms, including diarrhea, nausea, vomiting, and abdominal pain.
Indication
GLOPERBA 0.6 mg oral solution is indicated for prophylaxis of gout flares in adults. The safety and effectiveness of GLOPERBA for acute treatment of gout flares during prophylaxis has not been studied.
GLOPERBA is not an analgesic medication and should not be used to treat pain from other causes.
You are encouraged to report negative side effects of prescription drugs to the FDA. To report suspected adverse reactions, visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please see Full Prescribing Information for GLOPERBA.
References: 1. GLOPERBA [package insert]. Alpharetta, GA: Avion Pharmaceuticals, LLC; 2019. 2. US Food and Drug Administration. Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. https://www.accessdata.fda.gov/scripts/cder/ob/index.cfm. Updated January 2020. Accessed April 7, 2020. 3. Borstad GC, Bryant LR, Abel MP, Scroggie DA, Harris MD, Alloway JA. Colchicine for prophylaxis of acute flares when initiating allopurinol for chronic gouty arthritis. J Rheumatol. 2004;31(12):2429-2432.
References: 1. Eggebeen AT. Gout: an update. Am Fam Physician. 2007;76(6):801-808. 2. Bobulescu IA, Moe OW. Renal transport of uric acid: evolving concepts and uncertainties. Adv Chronic Kidney Dis. 2012;19(6):358-371. 3. Ragab G, Elshahaly M, Bardin T. Gout: an old disease in new perspective—a review. J Adv Res. 2017;8(5):495-511. 4. Finn WF. Kidney disease and gout: the role of the innate immune system. Open Urol Nephrol J. 2016;9(suppl 1):12-21. 5. Cronstein BN, Terkeltaub R. The inflammatory process of gout and its treatment. Arthritis Res Ther. 2006;8(1):S3. 6. Schlesinger N. Treatment of chronic gouty arthritis: it is not just about urate-lowering therapy. Semin Arthritis Rheum. 2012;42(2):155-165. 7. Bădulescu M, Macovei L, Rezuş E. Acute gout attack with normal serum uric acid levels. Rev Med Chir Soc Med Nat Iasi. 2014;118(4):942-945.
References: 1. Borstad GC, Bryant LR, Abel MP, Scroggie DA, Harris MD, Alloway JA. Colchicine for prophylaxis of acute flares when initiating allopurinol for chronic gouty arthritis. J Rheumatol. 2004;31(12):2429-2432. 2. Schlesinger N. Treatment of chronic gouty arthritis: it is not just about urate-lowering therapy. Semin Arthritis Rheum. 2012;42(2):155-165. 3. FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020;72(6):744-760. 4. GLOPERBA [package insert]. Alpharetta, GA: Avion Pharmaceuticals, LLC; 2019.
References: 1. GLOPERBA [package insert]. Alpharetta, GA: Avion Pharmaceuticals, LLC; 2019. 2. US Food and Drug Administration. Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. https://www.accessdata.fda.gov/scripts/cder/ob/index.cfm. Revised January 17, 2020. Accessed February 7, 2020. 3. Borstad GC, Bryant LR, Abel MP, Scroggie DA, Harris MD, Alloway JA. Colchicine for prophylaxis of acute flares when initiating allopurinol for chronic gouty arthritis. J Rheumatol. 2004;31:2429-2432. 4. Zhu Y, Pandya BJ, Choi HK. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007-2008. Am J Med. 2011;125:679-687. 5. Slobodnick A, Shah B, Pillinger MH, Krasnokutsky S. Colchicine: old and new. Am J Med. 2015;128(5):461-470.
References: 1. US Food and Drug Administration. Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. https://www.accessdata.fda.gov/scripts/cder/ob/index.cfm. Current through December 2020. Accessed December 7, 2020. 2. GLOPERBA [package insert]. Alpharetta, GA: Avion Pharmaceuticals, LLC; 2019.

